Background: CD19-targeted chimeric antigen receptor-engineered T cell(CAR-T) therapy with axicabtagene ciloleucel (axi-cel) is approved for patients (pts) with large cell lymphoma (LCL) [diffuse large cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL) and transformed follicular lymphoma (tFL)] after ≥ 2 lines of treatment. In addition to cytokine-release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), prolonged cytopenias may occur after axi-cel and can limit eligibility for subsequent treatments, especially clinical trials. In this study, we sought to determine the rate of clinically significant cytopenias and identify pre- and post- treatment factors associated with cytopenias after axi-cel.
Methods: Pts with DLBCL, PMBCL, or tFL who received axi-cel between Feb 2018 and Feb 2020 were included. We collected details about patients' demographics, disease characteristics, pre-CAR-T treatments, and post CAR-T specific toxicities (Table 1 footnote). We included details on blood counts, and transfusion/growth factor support needs from 30 days before leukapheresis (LA) until 30 days after treatment with axi-cel. We defined severe cytopenia if at least one of the following were present: (1) grade ≥ 3 thrombocytopenia (< 50x109/L), (2) neutropenia (< 1.0 x109/L), or (3) anemia (Hb < 8.0 g/dl) at day 30 after axi-cel, or (4) need for ≥ 2 doses of G-CSF, (5) ≥ 2 red blood cell (RBC) transfusions, or (6) ≥ 2 platelet (plt) transfusions between days 20-30 after axi-cel. Univariate and multivariable logistic regression was used to evaluate the association between each risk factor and the primary endpoint of severe cytopenia (Yes vs. No). For multivariable analysis, a novel high dimensional inference (HDI) approach was applied, which provides the de-biased estimates of the regression coefficients. HDI allows computation of adjusted odds ratios, confidence intervals and p values from high-dimensional datasets. This approach solves variable selection issues in CAR-T cell studies with small sample sizes and large number of predictors.
Results: 53 pts (41 DLBCL, 1 PMBCL, 11 tFL) received axi-cel during the study period. Median age was 63 (25-79) and 17 (32%) were female. Nineteen pts (36%) had bulky disease (> 5 cm) and 23 (43%) had elevated LDH (>210 U/L). Median number of therapies before CAR-T including stem cell transplant (SCT) and bridging therapy was 3 (2-9). Seventeen pts (32%) had a prior stem cell transplant (SCT) (16 autologous, 1 allogeneic) before CAR-T. Median time from last treatment to CAR-T was 4 weeks (0.3-130). Twenty six pts (49%) received bridging therapy between LA and lymphodepletion (LD). Following CAR-T, 37 (74%) patients achieved complete or partial remission (CR/PR) while 13 patients (26%) had progressive or stable disease (PD/SD). 45 (85%) pts developed CRS (grade 3-4: 5; 11% of all pts) and 31 (59%) developed ICANS (grade 3-4: 9; 15% of all pts).
Forty two (79%) patients had severe cytopenia as defined by one or more of the categories (Cat) listed in the methods; 24 (45%) by Cat 1 (thrombocytopenia), 19 (36%) by Cat 2 (neutropenia), 1 (2%) by Cat 3 (anemia), 10 (19%) by Cat 4 (GCS-F need), 11 (21%) by Cat 5 (RBC transfusion), or 15 (28%) by Cat 6 (plt transfusion). Severe cytopenia was more common in non-responders (SD/PD) vs. responders (PR/CR) (100% vs. 70%; P=.01).
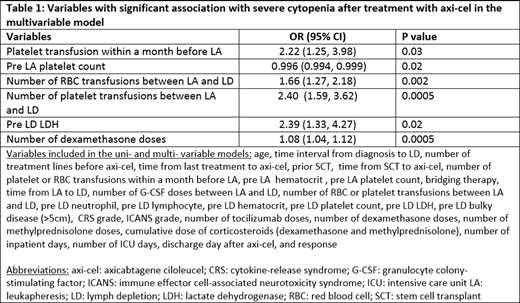
In the univariate analysis, lower pre-LA hematocrit (hct) (P=.005), pre-LD hct (P<.001), pre-LD plt count (P=.03), and pre-LD LDH (P=.02), and higher number of RBC transfusions between LA to LD (P=.03), number of dexamethasone (dexa) treatments after axi-cel (P=.03), and number of tocilizumab infusions (P=.04) were significantly associated with day 30 severe cytopenia. In the multivariable model (Table 1), plt transfusion within a month before LA, pre-LA plt count, number of RBC and plt transfusions between LA to LD, pre-LD LDH, and number of dexa treatments after CAR-T were significantly associated with severe cytopenia after axi-cel.
Conclusion: Severe cytopenia is common after axi-cel, -especially in non-responders in need of subsequent therapies. Our detailed analysis of pre- and post- CAR-T variables identified pre-CAR-T cytopenia and transfusion needs as well as post-CAR-T steroid use as potential predictors of day 30 severe cytopenia in pts with LCL receiving axi-cel.
Gauthier:JMP, Eusapharma, Multerra Bio: Honoraria. Cassaday:Amgen: Consultancy, Research Funding; Vanda Pharmaceuticals: Research Funding; Pfizer: Honoraria, Research Funding; Seattle Genetics: Current Employment, Current equity holder in publicly-traded company; Kite/Gilead: Consultancy, Research Funding; Merck: Research Funding. Kiem:Magenta Therapeutics, CSL,Homology Medicines, Vor Biopharma , Enochian, Umoja, Rocket Pharma: Consultancy. Lynch:TG Therapeutics: Research Funding; Takeda: Research Funding; MorphoSys: Consultancy; Genentech: Research Funding; Cyteir: Research Funding; Bayer: Research Funding; Rhizen Pharmaceuticals: Research Funding; Incyte: Research Funding; Juno Therpeutics: Research Funding. Ujjani:Atara: Consultancy, Honoraria; Gilead/Kite: Consultancy, Research Funding; Verastem Oncology: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; MorphoSys: Consultancy. Smith:AstraZeneca: Research Funding; Acerta Pharma BV: Research Funding; Beigene: Consultancy; Millenium/Takeda: Consultancy; AstraZeneca: Consultancy; Portola: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Incyte: Research Funding; Ignyta: Research Funding; Genentech: Research Funding; De Novo Biopharma: Research Funding; Ayala: Research Funding; Bristol Meyers Squibb: Research Funding; Bayer: Research Funding; Karyopharm: Consultancy. Gopal:Seattle Genetics; Janssen; Takeda; IgM Bio; IMab Bio; BMS; Astra Zeneca; Merck; Gilead: Research Funding; Seattle Genetics; Janssen; IMab Bio; TG Therapeutics; Astra Zeneca; Merck; Gilead; ADC Therapeutics; Nurix; TG therapeutics, Cellectar; Actinium: Consultancy; imab bio, takeda,astrazeneca,gilead: Research Funding; IgM bio, BMS, merck: Research Funding. Till:Mustang: Patents & Royalties, Research Funding. Turtle:Kite/Gilead: Consultancy; Humanigen: Consultancy; Physician Education Resource: Consultancy; Century Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Research Funding; PACT Pharma: Consultancy; Allogene: Consultancy; T-CURX: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Arsenal Bio: Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Caribou Biosciences: Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Eureka Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Precision Biosciences: Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Consultancy, Research Funding; Juno/BMS: Patents & Royalties, Research Funding; Novartis: Consultancy. Maloney:Genentech: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Current equity holder in publicly-traded company, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Bioline Rx: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: Patents are pending, but not issued, licensed, no royalties, no licensees., Research Funding; MorphoSys: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria. Shadman:Mustang Bio, Celgene, Pharmacyclics, Gilead, Genentech, Abbvie, TG therapeutics, Beigene, Astra Zeneca, Sunesis, Beigene: Research Funding; Abbvie, Genentech, Astra Zeneca, Sound Biologics , Pharmacyclics, Verastem, ADC therapeutics, Beigene, Cellectar, BMS, Morphosys and Atara Biotherapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.